Abstract
Various chemotherapy regimens attempted however have been evaluated these treatment e not effective in chronic myeloid leukemia myeloid blastic phase (CML-MBP). Imatinib treatment in this subset of patients were also not very promising due to low response rates, short response duration, and transformation to BC. Some studies showed that nilotinib or dasatinib were superior to imatinib in terms of rapid time to response?? and higher molecular response in newly diagnosed CML patients . It can be translated to CML-MBP. We evaluated 2nd generation TKI, nilotinib and high-dose daunorubicin induction chemotherapy combination if to attempt an improvement in the response rate and survival in patients with CML-MBP or acute myeloid leukemia with BCR/ABL1 (AML-BCR/ABL1).
The primary end point of the study was complete remission (CR) rate after induction chemotherapy (IC). Patients received cytarabine 200 mg/m2/day by continuous IV infusion over 24 hours daily for 7 days (D 1-7) along with daunorubicin 90 mg/m2/day IV daily for 3 days (D 1-3). Nilotinib 400mg bid PO was added without interruption from D8 of induction chemotherapy until allogeneic hematopoietic cell transplantation (alloHCT) or during 2 years. Re-induction chemotherapy was given as cytarabine 200 mg/m2/day by continuous iv infusion over 24 hours daily for 5 days (D 1-5) plus daunorubicin 45 mg/m2/day iv daily for 2 days (D 1-2) when D14 bone marrow blasts exceeded 5%. Four courses of high-dose cytarabine (Cytarabine 3 g/m2) were administered in 3-hour IV infusion every 12 hours on days 1, 3, and 5 (a total of six doses per course). This prospective phase II study was early terminated due to slow enrollment.
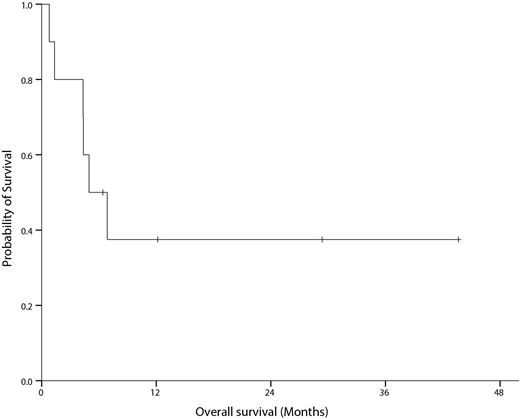
A total of 10 (6 male & 4 female) patients were enrolled. Six patients were diagnosed as CML-MBP and 4 patients as AML-BCR/ABL1 (all these patients will be presented as CML-MBP). Median age CML-MBP patients was 50.9 (22.5-63.0) years. Five patients had received prior therapies (low-dose cytarabine in 1, imatinib in 2 and dasatinib in 2 patients). Median time from CML-MBP diagnosis to induction chemotherapy was 3.12 (0-3.12) months. All patients received IC. Nilotinib was interrupted temporarily during IC in 5 patients (hyperbilirubin emia in 2 pts?, cytopenia in 1, rash in 1 and poor general condition in 1 patient). Nine patients showed bone marrow (BM) blast<5% in early evaluation. CR was achieved in all (CR in 9 patients and CRi in 1) patients. One patient who had achieved CRi died of pneumonia without full hematological recovery during IC. Molecular response (MRIS) at the time of CR was not-evaluable in 1, MR1.0 in 3, MR2.0 in 2, MR3.0 in 2 and MR4.5 in 2 patients. Eight patients received 1 or 2 cycles of consolidation and 1 patients died during consolidation therapy. Five patients proceeded to alloHCT after consolidation. Two more (1 by relapse after alloHCT and 1 by graft-versus-host disease) patients died after alloHCT. Two patients died after alloHCT (1 by relapse and 1 after stopping nilotinib by toxicities). Median overall survival was 5.0 (95% CI, 1.5-8.4) months. Four patients are still alive (3 patients after alloHCT and 1 patient without alloHCT; 6.4+, 12.2+, 29.4+ and 43.6 months).
In conclusion, nilotinib combined with IC is feasible and can be a good bridging therapy for these extremely rare CML-MBP or AML-BCR/ABL1 if they are IC-eligible although this conclusion is very limited and should be confirmed by large scale studies because of the small sample size of this study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal